What happens at the atomic level of a battery?
Explain what’s happening at the atomic level when a battery is used to turn on a light bulb.

Overview
Students think through key ideas of atomic theory. For example, some atoms hold on to electrons better than others and that like charges repel and opposite charges attract. After exploring key concepts, students decide which statements best explain the flow of electric current through a conductor.
Instructions
What you'll need
- "Which statement is the most relevant?" worksheet, one per student
- "What happens at the atomic level?" slideshow
- Digital projector and screen
- Organize your students into small groups (2-3 students).
- Open "What happens at the atomic level?" slideshow, display slide 2 and the image of the closed circuit. Inform your students that their challenge is to explain what’s happening at the atomic level when a battery is used to power a light bulb.
- Ask groups to discuss their initial thoughts about what’s happening at the atomic level in the diagram. Invite groups to share their thinking with the class.
- Provide each student with a copy of "Which statement is the most relevant?" worksheet. Explain that while all the statements on the worksheet are technically true, only some are relevant to their explanation of the closed circuit.
- Prompt groups to indicate on the worksheet which statements might be relevant when explaining what happens at the atomic level when a battery is used to power a light bulb.
- Similarly, ask groups to indicate which statements might be accurate but irrelevant to the explanation.
- After groups have had a few moments to explore the statements, display slide 3. Discuss the diagrams with your students, inviting them to suggest how the ideas in the diagrams might be used to explain what happens at the atomic level when a battery is used to power a light bulb. Encourage groups to use these ideas to revisit the statements on their worksheets.
- Invite groups to share their relevant and non-relevant statements with the class. Discuss any differences in students’ answers.
- Conclude the activity by asking individual students to select the three most relevant statements. Invite students to share their decisions and thinking with the class.
Curriculum Fit
Grade 8 Science
Big idea
- The behaviour of matter can be explained by the kinetic molecular theory and atomic theory.
Content
- Atomic theory and models
- Models can be used to represent:
- The arrangement and motion of particles in different phases
- The arrangement of and forces that bind protons, neutrons, and electrons in an atom
- The quarks and leptons in protons, neutrons, and electrons
- Models can be used to represent:
- Protons, neutrons, and quarks
- Protons and neutrons (made of quarks) are held together in the nucleus by a strong nuclear force
Curricular competencies
Questioning and predicting
- Demonstrate a sustained intellectual curiosity about a scientific topic or problem of personal interest
- Make observations aimed at identifying their own questions about the natural world
- Identify a question to answer or a problem to solve through scientific inquiry
Processing and analyzing scientific information
- Use scientific understandings to identify relationships and draw conclusions
- Construct and use a range of methods to represent patterns or relationships in data, including tables, graphs, keys, models, and digital technologies as appropriate
Evaluating
- Demonstrate an understanding and appreciation of evidence (qualitative and quantitative)
Applying and innovating
- Contribute to care for self, others, community, and world through personal or collaborative approaches
- Transfer and apply learning to new situations
Communicating
- Communicate ideas, findings, and solutions to problems, using scientific language, representations, and digital technologies as appropriate
Assessments
Assess your students’ abilities to:
- Develop and support effective scientific explanations.
- Effectively describe the arrangement and motion of particles in different phases.
Teaching Notes
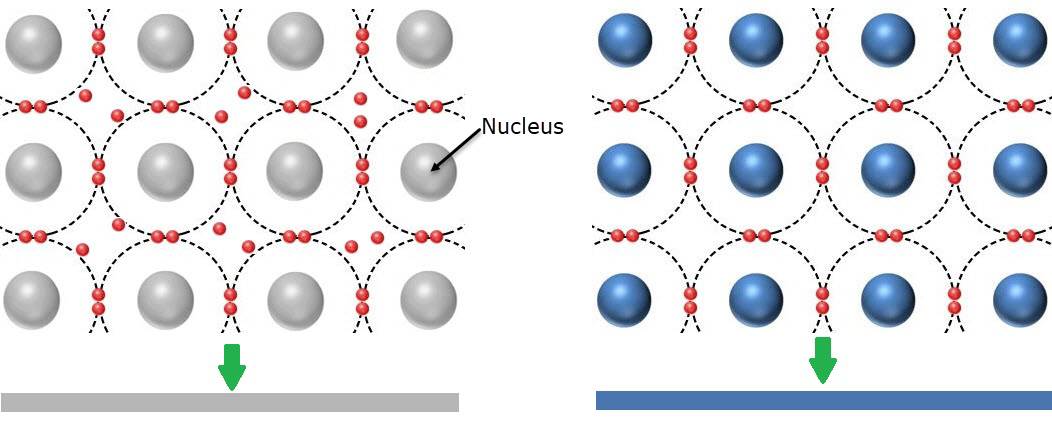
This is image compares a Bohr type model of a conductor and insulator. The key idea is that the conductor has free electrons that are loosely held by the nucleus, and the insulator is made of a compound that has tightly held electrons in an organized lattice matrix.







