Atomic models and electricity
Get your students thinking critically about the relationships between atomic models and electricity.
- Grade 8
- 4 activities
- 2 hours
Big idea
The behaviour of matter can be explained by atomic theory.
Learning objectives
Students will understand how atomic theory can be used to explain and understand the generation and movement of electricity.
Activities
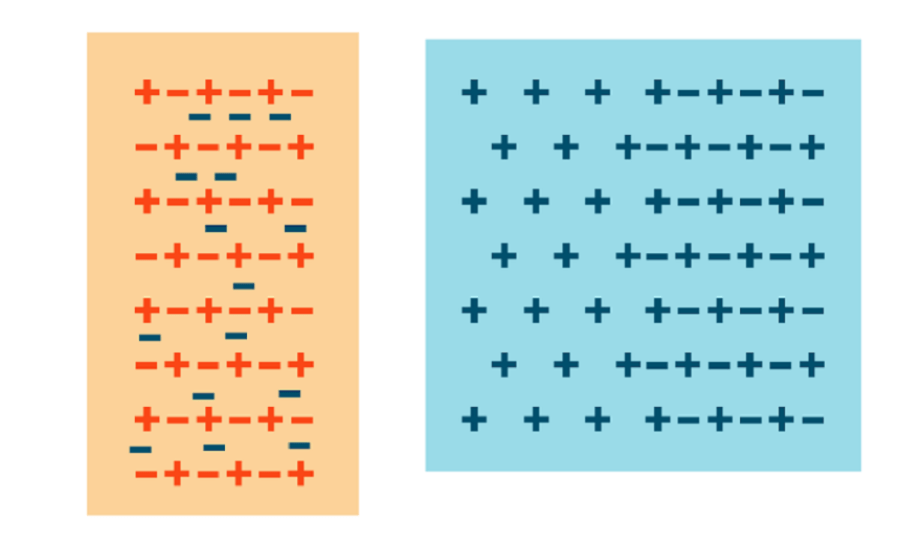
What’s the charge?
Develop effective scientific explanations to learn more about static electricity.
BC curriculum fit
Grade 8 Science
Big idea
- The behaviour of matter can be explained by the kinetic molecular theory and atomic theory.
Content
- Atomic theory and models
- Models can be used to represent:
- The arrangement and motion of particles in different phases
- The arrangement of and forces that bind protons, neutrons, and electrons in an atom
- The quarks and leptons in protons, neutrons, and electrons
- Models can be used to represent:
- Protons, neutrons, and quarks
- Protons and neutrons (made of quarks) are held together in the nucleus by a strong nuclear force
Curricular competencies
Questioning and predicting
- Demonstrate a sustained intellectual curiosity about a scientific topic or problem of personal interest
- Make observations aimed at identifying their own questions about the natural world
- Identify a question to answer or a problem to solve through scientific inquiry
Processing and analyzing scientific information
- Use scientific understandings to identify relationships and draw conclusions
- Construct and use a range of methods to represent patterns or relationships in data, including tables, graphs, keys, models, and digital technologies as appropriate
Evaluating
- Demonstrate an understanding and appreciation of evidence (qualitative and quantitative)
Applying and innovating
- Contribute to care for self, others, community, and world through personal or collaborative approaches
- Transfer and apply learning to new situations
Communicating
- Communicate ideas, findings, and solutions to problems, using scientific language, representations, and digital technologies as appropriate
Assessments
All matter is made up of atoms, which are themselves made up of charged particles. Atoms have a nucleus consisting of neutrons and protons. They also have a surrounding "shell" that is made up of electrons. Typically, matter is neutrally charged, meaning that the number of electrons and protons are the same. If an atom has more electrons than protons, it is negatively charged. If it has more protons than electrons, it is positively charged.
Background info
All matter is made up of atoms, which are themselves made up of charged particles. Atoms have a nucleus consisting of neutrons and protons. They also have a surrounding "shell" that is made up of electrons. Typically, matter is neutrally charged, meaning that the number of electrons and protons are the same. If an atom has more electrons than protons, it is negatively charged. If it has more protons than electrons, it is positively charged.